
Long ago, alchemists concerned themselves with refining base materials like lead into substances with higher spiritual value, like gold. I argue that alchemists still lurk among us and that the alchemists are the plants.1 Plants take air and water, combine them using energy from the sun, and make a substance holding energy that powers life for all organisms on earth.
How much air and water are really in this miracle substance, you might ask. A lot. After you dehydrate a plant, 90% of what is left is from the air (all of the carbon, C, and all of the oxygen, O). Another 6% of a plant’s dry water is H’s from the water. That leaves only 4% of a plant’s dry matter from the soil. That is, even if you grew a huge plant in a pot, you would hardly diminish the soil because the plant’s body is made mostly from air and water.
Please read on. This alchemy is one of the most astonishing accomplishments on earth. And without it, there would be no one here to be astonished.
What are the basic processes of capturing and using energy?
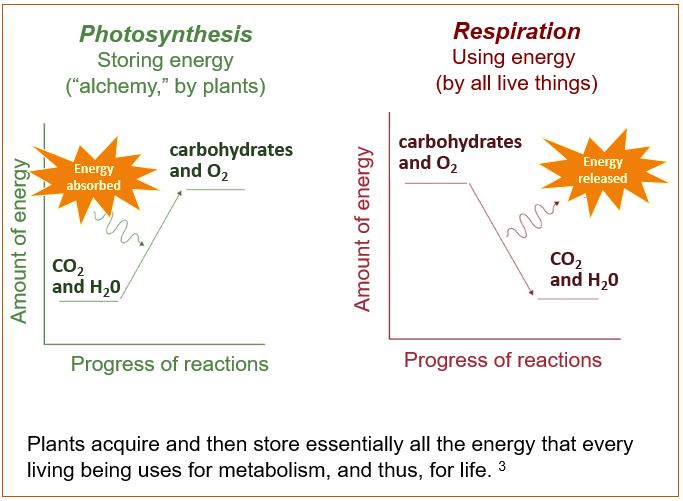
Photosynthesis is the process of capturing energy and storing it in bonds in carbohydrates. Plants take carbon dioxide (CO2) from the air and hydrogen (H) from water, then, presto-chango, make a carbohydrate (initially a sugar, that can be switched around to starch, cellulose, and all manners of compounds). The net reaction of photosynthesis (below) looks so simple, but it hides that there are at least fifty intermediate reactions.
6 CO2 + 6 H2O + light energy –> C6H12O6 (a carbohydrate) + 6 O2
The end-product, the carbohydrate, has much more energy embedded in it than the CO2 and the H2O did in the first place. This extra energy is stored its chemical the bonds between its C’s, H’s, and O’s. Note that O2 is another output of photosynthesis.
In respiration2, living things use the energy from these bonds, along with some O2 (unless we are fermenters) to power every process of our lives, like cell division, growth, movement, and digestion. In respiration, unless we are fermenters, we have to use O2 during respiration. It doesn’t surprise me to remember that my brain (and the rest of me) needs oxygen to stay alive, and this is why.
Who cares?
Everything has to respire to live, but photosynthetic organisms are the only ones that can make the carbohydrates that, ultimately, all of us respire. They make us, and all other life, possible. They deserve ballads, tributes, and gratitude.
If they ever stopped photosynthesizing, we, and everything else, would die. But that’s not going to happen.
What is happening is that we are changing the atmosphere, soil, and climate, all of which affect plant health and vigor. Understanding how to keep plants healthy is essential for food and fiber security, and to help come up with strategies to control CO2 in the atmosphere. Understanding and appreciating plants is also an ethical obligation as we assess and manage our impacts on the universe.
How is energy captured?

How does this alchemy actually work? We can answer that at many levels, and they all have to do with how structures or shapes affect function. The leaves in this photo show an adaptation on rainforest understory that minimizes the overlap of leaves within one plant, to make that plant’s capture of light energy more efficient. There are so many “strategies” employed, at so many levels. The study of these is a branch of physiological ecology, which is a subject researched and taught at colleges and universities around the world.
Some of the structure/function questions related to photosynthesis are these. What are the shapes of the biochemical molecules, and where are they held with respect to one another? What is the shape of the organelle (the chloroplast) within the cell where different reactions take place? Where in the cell are the chloroplasts? Where in the leaf are the cells that perform photosynthesis? What is the size and orientation of the leaves? How many layers of leaves does a plant have? To what extent are the leaves competing with leaves of other plants for that solar energy?
The actual “catching” of the solar energy occurs first by pigments that have long pleated chains of C’s, H’s, and O’s that function as antennas. When a photon of an appropriate energy level hits a chain, the chain will vibrate. Different pigments vibrate when photons of different light energies strike them.
The vibration of one antenna doesn’t carry much energy, but these antennas are held in arrays that together harvest sufficient vibrational energy to knock an electron off an H2O and into a higher energy state. That action results in splitting the H20 molecule into H’s (later used as part of the carbohydrate that is formed) and O2 (that is released).
What happens to the energy?
First, we already said that the energy goes into the formation of carbohydrates, and that there are some fifty separate chemical reactions along the way to take six CO2 and six H2O molecules together with this energy harvested from the light, to end up with one carbohydrate (C6H12O6) and six O2 molecules.
Then, analogous to how we use energy in a battery, organisms “discharge” the carbohydrate bond energy by breaking the carbohydrate down into compounds that have less and less energy in them, until finally, there is only CO2 and H2O again. Organisms control the release of the energy to fuel metabolism–everything that requires chemical energy for life.
If you eat plant material, you will break down the carbohydrates for energy. If you eat cheese or fish, though, you are breaking down energy products that other organisms (here, a cow or a fish) captured from a plant, or from something they ate that originated in a plant. There is no other source of chemical energy that any of us can access in a meaningful amount.4
An aside is that plants do two quite distinct things with their carbohydrates. They use them as an energy source like we do, but they also use them as a building material, such as to make structural material (e.g., analogous to our skeletons), like wood, or fibers in cotton, flax, or hemp. Some of the bonds in these structural materials are switched around, making wood or fiber hard for other organisms to digest. Even so, this structural material still has some energy stored In it, and some decomposers have evolved enzymes to break it down and release its energy for their use.
“Fossil fuels” are mostly old structural material–dead plants with some energy still tied up in their bonds. When we burn fossil fuels, we get the energy out.

Similarly, when we burn a log, we are releasing energy that was locked into bonds in the structural material (the wood). Say the tree grew from 1980 to 2010. The heat you get out from the log came from the sun’s energy that was stored in the structure of the wood, some that reached the tree in 1980, some in 1981, some in 1982, and so on. That heat is, literally, the energy from the sun in each of those thirty years.
Above, I mentioned that plants use the carbohydrates as an energy source, as we do. I didn’t mention lips (fats, oils). Plants can use some of their energy to further concentrate the energy storage in the carbon-based material by making these lipids. The same weight of a lipid has about 2.5 times more energy in it than starch does. Many seeds have a lot of lipid in them, not surprisingly: the plant has evolved to put this high-energy-per-weight material in seeds, whose dispersal would be hindered if they were too large or heavy.
Alchemists among us

And so this is why I venerate plants. They make a solid material, almost from thin air. It’s a material upon which all life depends, the energy-rich material called carbohydrates. To me, these carbohydrates are immeasurably more valuable than gold.
Perhaps it is more fitting to say, not that these alchemists lurk among us, but that by the grace of these alchemists, we lurk among them.
* A post is in my series Wow Trees.
1 I am writing about photosynthetic organisms, and then referring to them, for simplicity, as plants. Plants includes familiar ones like geraniums, lilies, pine trees, mosses, and ferns, as well as less familiar plants like liverworts. There are also photosynthetic organism within the taxonomic groups of algae (which includes seaweed), Euglena, and bacteria.
2 This use of the term respiration is not the same use as the word related to breathing.
3 Modified from www.chemiwiki.ucdavis.edu
4 With the exception of extremophiles, organisms that may use H2S rather than H2O, and absorb energy from earth-generated heat such as from hot springs in deep waters or other locations where the sun’s light energy doesn’t reach.
Leave a comment